not been investigated in patients with compromised corneas (particularly in patients with low endothelial cell count). Specifically, patients wearing contact lenses have not been studied and careful monitoring of these patients when using brinzolamide is recommended, since carbonic anhydrase inhibitors may affect corneal hydration and wearing contact lenses might increase the risk for the cornea. Careful monitoring of patients with compromised corneas, such as patients with diabetes mellitus or corneal dystrophies, is recommended. SIMBRINZA may be used while wearing contact lenses with careful monitoring (see below under 'Benzalkonium chloride').
Brimonidine tartrate may cause ocular allergic reactions. If allergic reactions are observed, treatment should be discontinued. Delayed ocular hypersensitivity reactions have been reported with brimonidine tartrate, with some reported to be associated with an increase in IOP.
The potential effects following cessation of treatment with SIMBRINZA have not been studied. While the duration of IOP-lowering effect for SIMBRINZA has not been studied, the IOP-lowering effect of brinzolamide is expected to last for 5-7 days. The IOP-lowering effect of brimonidine may be longer.
Systemic effectsSIMBRINZA contains brinzolamide, a sulphonamide inhibitor of carbonic anhydrase and, although administered topically, is absorbed systemically. The same types of adverse reactions that are attributable to sulphonamides may occur with topical administration. If signs of serious reactions or hypersensitivity occur, the use of this medicinal product should be discontinued.
Cardiac disordersFollowing administration of SIMBRINZA, small decreases in blood pressure were observed in some patients. Caution is advised when using medicinal products such as antihypertensives and/or cardiac glycosides concomitantly with SIMBRINZA or in patients with severe or unstable and uncontrolled cardiovascular disease (see section 4.5)
SIMBRINZA should be used with caution in patients with depression, cerebral or coronary insufficiency, Raynaud's phenomenon, orthostatic hypotension or thromboangiitis obliterans.
Acid/base disturbancesAcid-base disturbances have been reported with oral carbonic anhydrase inhibitors. SIMBRINZA contains brinzolamide, an inhibitor of carbonic anhydrase, and although administered topically, is absorbed systemically. The same types of adverse reactions that are attributable to oral carbonic inhibitors (i.e., acid-base disturbances) may occur with topical administration (see section 4.5).
Use with caution in patients with risk of renal impairment because of the possible risk of metabolic acidosis. SIMBRINZA is contraindicated in patients with severe renal impairment (see section 4.3).
Hepatic impairmentSIMBRINZA has not been studied in patients with hepatic impairment; caution should be used in treating such patients (see section 4.2).
Mental alertnessOral carbonic anhydrase inhibitors may impair the ability to perform tasks requiring mental alertness and/or physical coordination in elderly patients. SIMBRINZA is absorbed systemically and therefore this may occur with topical administration (see section 4.7).
Benzalkonium chlorideSIMBRINZA contains benzalkonium chloride which may cause eye irritation and is known to discolour soft contact lenses. Contact with soft contact lenses should be avoided. Patients must be instructed to remove contact lens prior to application of SIMBRINZA and wait at least 15 minutes before reinsertion.
Benzalkonium chloride has also been reported to cause punctate keratopathy and/or toxic ulcerative keratopathy. Close monitoring is required with frequent or prolonged use.
Paediatric populationThe safety and efficacy of SIMBRINZA in children and adolescents aged 2 to 17 years has not been established. Symptoms of brimonidine overdose (including loss of consciousness, hypotension, hypotonia, bradycardia, hypothermia, cyanosis and apnoea) have been reported in neonates and infants receiving brimonidine eye drops as part of medical treatment of congenital glaucoma. SIMBRINZA is therefore contraindicated in children below 2 years of age (see section 4.3).
Treatment of children 2 years and above (especially in those in the 2-7 age range and/or weighing < 20 kg) is not recommended because of the potential for central nervous system-related side effects (see section 4.9).
4.5 Interaction with other medicinal products and other forms of interactionNo specific drug interaction studies have been performed with SIMBRINZA.
SIMBRINZA is contraindicated in patients receiving monoamine oxidase inhibitors and patients on antidepressants which affect noradrenagic transmission (e.g. tricyclic antidepressants and mianserin), (see section 4.3). Tricyclic antidepressants may blunt the ocular hypotensive response of SIMBRINZA.
Caution is advised due to the possibility of an additive or potentiating effect with CNS depressants (e.g. alcohol, barbiturates, opiates, sedatives, or anaesthetics).
No data on the level of circulating catecholamines after SIMBRINZA administration are available. Caution, however, is advised in patients taking medicinal products which can affect the metabolism and uptake of circulating amines (e.g. chlorpromazine, methylphenidate, reserpine, serotonin-norepinephrine reuptake inhibitors).
Alpha adrenergic agonists (e.g., brimonidine tartrate), as a class, may reduce pulse and blood pressure. Following administration of SIMBRINZA, small decreases in blood pressure were observed in some patients. Caution is advised when using medicinal products such as antihypertensives and/or cardiac glycosides concomitantly with SIMBRINZA.
Caution is advised when initiating (or changing the dose of) a concomitant systemic medicinal products (irrespective of pharmaceutical form) which may interact with α-adrenergic agonists or interfere with their activity i.e. agonists or antagonists of the adrenergic receptor (e.g. isoprenaline, prazosin).
Brinzolamide is a carbonic anhydrase inhibitor and, although administered topically, is absorbed systemically. Acid-base disturbances have been reported with oral carbonic anhydrase inhibitors. The potential for interactions must be considered in patients receiving SIMBRINZA.
There is a potential for an additive effect on the known systemic effects of carbonic anhydrase inhibition in patients receiving an oral carbonic anhydrase inhibitor and topical brinzolamide. The concomitant administration of SIMBRINZA and oral carbonic anhydrase inhibitors is not recommended.
The cytochrome P-450 isozymes responsible for metabolism of brinzolamide include CYP3A4 (main), CYP2A6, CYP2B6, CYP2C8 and CYP2C9. It is expected that inhibitors of CYP3A4 such as ketoconazole, itraconazole, clotrimazole, ritonavir and troleandomycin will inhibit the metabolism of brinzolamide by CYP3A4. Caution is advised if CYP3A4 inhibitors are given concomitantly. However, accumulation of brinzolamide is unlikely as renal elimination is the major route. Brinzolamide is not an inhibitor of cytochrome P-450 isozymes.
4.6 Fertility, pregnancy and lactationPregnancyThere are no or limited amount of data from the use of SIMBRINZA in pregnant women. Brinzolamide was teratogenic in rats, but not rabbits, following systemic administration. Animal studies with oral brimonidine do not indicate direct harmful effects with respect to reproductive toxicity. In animal studies, brimonidine crossed the placenta and entered into the fetal circulation to a limited extent. SIMBRINZA is not recommended during pregnancy and in women of child bearing potential not using contraception.
Breast-feedingIt is unknown whether topical SIMBRINZA is excreted in human milk. Available pharmacodynamic/toxicological data in animals have shown that following oral administration, minimal levels of brinzolamide are excreted in breast milk. Brimonidine administered orally is excreted in breast milk. SIMBRINZA should not be used by women nursing infants.
FertilityNonclinical data do not show any effects of brinzolamide or brimonidine on fertility. There are no data on the effect of topical ocular administration of SIMBRINZA on human fertility.
4.7 Effects on ability to drive and use machinesSIMBRINZA has a moderate influence on the ability to drive and use machines.
SIMBRINZA may cause dizziness, fatigue and/or drowsiness, which may impair the ability to drive or use machines.
Temporary blurred vision or other visual disturbances may affect the ability to drive or use machines. If blurred vision occurs at instillation the patient must wait until the vision clears before driving or using machines.
Oral carbonic anhydrase inhibitors may impair the ability of elderly patients to perform tasks requiring mental alertness and/or physical coordination (see section 4.4).
4.8 Undesirable effectsSummary of the safety profileIn clinical trials involving SIMBRINZA dosed twice-daily the most common adverse reactions were ocular hyperaemia and ocular allergic type reactions occurring in approximately 6-7% of patients, and dysgeusia (bitter or unusual taste in the mouth following instillation) occurring in approximately 3% of patients. The safety profile of SIMBRINZA was similar to that of the individual components (brinzolamide 10 mg/mL and brimonidine 2 mg/mL).
Tabulated summary of adverse reactionsThe following adverse reactions have been reported during clinical studies with SIMBRINZA twice-daily dosing and during clinical studies and post-marketing surveillance with the individual components brinzolamide and brimonidine. They are classified according to the subsequent convention: very common (≥ 1/10), common (≥ 1/100 to <1/10), uncommon (≥1/1,000 to <1/100), rare (≥1/10,000 to <1/1,000) and very rare (<1/10,000). Within each frequency-grouping, adverse reactions are presented in order of decreasing seriousness.
System Organ Classification
| Adverse reactions
|
Infections and infestations
| Uncommon: nasopharyngitis2, pharyngitis2, sinusitus2
Not known: rhinitis2
|
Blood and lymphatic system disorders
| Uncommon: red blood cell decreased2, blood chloride increased2
|
Immune system disorders
| Uncommon: hypersensitivity3
|
Psychiatric disorders
| Uncommon: apathy2, depression2,3, depressed mood2, insomnia1, libido decreased2, nightmare2, nervousness2
|
Nervous system disorders
| Common: somnolence1, dizziness3, dysgeusia1
Uncommon: headache1, motor dysfunction2, amnesia2, memory impairment2, paraesthesia2
Very rare: syncope3
Not known: tremor2, hypoaesthesia2, ageusia2
|
Eye disorders
| Common: eye allergy1, keratitis1, eye pain1, ocular discomfort1, blurred vision1, abnormal vision3, ocular hyperaemia1, conjunctival blanching3
Uncommon: corneal erosion1, corneal oedema2, blepharitis1, corneal deposits (keratic precipitates) 1, conjunctival disorder (papillae) 1, photophobia1, photopsia2, eye swelling2, eyelid oedema1, conjunctival oedema1, dry eye1, eye discharge1, visual acuity reduced2, lacrimation increased1, pterygium2, erythema of eyelid1, meibomianitis2, diplopia2, glare2, hypoaesthsia eye2, scleral pigmentation2, subconjunctival cyst2,abnormal sensation in eye1, asthenopia1
Very rare: uveitis3, miosis3
Not known: visual disturbances2, madarosis2
|
Ear and labyrinth disorders
| Uncommon: vertigo1, tinitus2
|
Cardiac disorders
| Uncommon: cardio-respiratory distress2, angina pectoris2, arrhythmia3, palpitations2,3, heart rate irregular2, bradycardia2,3, tachycardia3
|
Vascular disorders
| Uncommon: hypotension1
Very rare: hypertension3
|
Respiratory, thoracic and mediastinal disorders
| Uncommon: dyspnoea2, bronchial hyperactivity2, pharyngolaryngeal pain2, dry throat1, cough2, epistaxis2, upper respiratory tract congestion2, nasal congestion1, rhinorrhea2, throat irritation2, nasal dryness1, postnasal drip1, sneezing2
Not known: asthma2
|
Gastrointestinal disorders
| Common: dry mouth1
Uncommon: dyspepsia1, oesophagitis2, abdominal discomfort1, , diarrhoea2, vomiting2, nausea2, frequent bowel movements2, flatulence2, hypoaesthesia oral2, paraesthesia oral1
|
Hepatobiliary disorders
| Not known: liver function test abnormal2
|
Skin and subcutaneous tissue disorders
| Uncommon: dermatitis contact1, urticaria2, rash2, rash maculo-papular2, pruritus generalized2, alopecia2, skin tightness2
Not known: face oedema3, dermatitis2,3, erythema2,3
|
Musculoskeletal and connective tissue disorders
| Uncommon: back pain2, muscle spasms2, myalgia2
Not known: arthralgia2, pain in extremity2
|
Renal and urinary disorders
| Uncommon: renal pain2
Not known: pollakiuria2
|
Reproductive system and breast disorders
| Uncommon: erectile dysfunction2
|
General disorders and administration site conditions
| Uncommon: pain2, chest discomfort2, feeling abnormal2, feeling jittery2, irritability2, medication residue1
Not known: chest pain2, peripheral oedema2,3
|
1 adverse reaction observed with Simbrinza
2 additional adverse reaction observed with brinzolamide monotherapy
3 additional adverse reaction observed with brimonidine monotherapy
Description of selected adverse reactionsDysgeusia was the most common systemic adverse reaction associated with the use of SIMBRINZA (3.4%). It is likely to be caused by passage of the eye drops in the nasopharynx via the nasolacrimal canal and is mainly attributable to brinzolamide component of SIMBRINZA. Nasolacrimal occlusion or gently closing the eyelid after instillation may help reduce the occurrence of this effect (see section 4.2).
SIMBRINZA contains brinzolamide which is a sulphonamide inhibitor of carbonic anhydrase with systemic absorption. Gastrointestinal, nervous system, haematological, renal and metabolic effects are generally associated with systemic carbonic anhydrase inhibitors. The same type of adverse reactions attributable to oral carbonic anhydrase inhibitors may occur with topical administration.
Adverse reactions commonly associated with the brimonidine component of SIMBRINZA include the development of ocular allergic type reactions, fatigue and/or drowsiness, and dry mouth. The use of brimonidine has been associated with minimal decreases in blood pressure. Some patients who dosed with SIMBRINZA experienced decreases in blood pressure similar to those observed with the use of brimonidine as monotherapy.
Reporting of suspected adverse reactionsReporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system listed below:
United KingdomYellow Card Scheme
Website:
www.mhra.gov.uk/yellowcardIrelandIMB Pharmacovigilance,
Earlsfort Terrace, IRL - Dublin 2; Tel: +353 1 6764971
Fax: +353 1 6762517. Website:
www.imb.ie; e-mail:
imbpharmacovigilance@imb.ieMaltaADR Reporting
The Medicines Authority
Post-Licensing Directorate
203 Level 3, Rue D'Argens
GŻR-1368 Gżira
Website:
www.medicinesauthority.gov.mte-mail:
postlicensing.medicinesauthority@gov.mt4.9 OverdoseIf overdose with SIMBRINZA occurs treatment should be symptomatic and supportive. The patient's airway should be maintained.
Due to the brinzolamide component of SIMBRINZA, electrolyte imbalance, development of an acidotic state, and possible nervous system effects may occur. Serum electrolyte levels (particularly potassium) and blood pH levels must be monitored.
There is very limited information regarding accidental ingestion with the brimonidine component of SIMBRINZA in adults. The only adverse event reported to date was hypotension. It was reported that the hypotensive episode was followed by rebound hypertension.
Oral overdoses of other alpha-2-agonists have been reported to cause symptoms such as hypotension, asthenia, vomiting, lethargy, sedation, bradycardia, arrhythmias, miosis, apnoea, hypotonia, hypothermia, respiratory depression and seizure.
Paediatric populationSerious adverse effects following inadvertent ingestion with the brimonidine component of SIMBRINZA by paediatric subjects have been reported. The subjects experienced symptoms of CNS depression, typically temporary coma or low level of consciousness, lethargy, somnolence, hypotonia, bradycardia, hypothermia, pallor, respiratory depression and apnoea, and required admission to intensive care with intubation if indicated. All subjects were reported to have made a full recovery, usually within 6-24 hours.
5. Pharmacological properties5.1 Pharmacodynamic propertiesPharmacotherapeutic group: not yet assigned ATC code: not yet assigned
Mechanism of actionSIMBRINZA contains two active substances: brinzolamide and brimonidine tartrate. These two components lower intraocular pressure (IOP) in patients with open-angle glaucoma (OAG) and ocular hypertension (OHT) by suppressing the formation of aqueous humour from the ciliary process in the eye. Although both brinzolamide and brimonidine lower IOP by suppressing aqueous humour formation, their mechanisms of action are different.
Brinzolamide acts by inhibiting the enzyme carbonic anhydrase (CA-II) in the ciliary epithelium that reduces the formation of bicarbonate ions with subsequent reduction in sodium and fluid transport across the ciliary epithelium, resulting in decreased aqueous humour formation. Brimonidine, an alpha-2 adrenergic agonist, inhibits the enzyme adenylate cyclase and suppresses the cAMP-dependent formation of aqueous humour. Additionally, administration of brimonidine results in an increase in uveoscleral outflow.
Pharmacodynamic effectsClinical efficacy and safetyIn a 6-month, controlled, contribution of elements clinical study enrolling 560 patients with open-angle glaucoma (including pseudoexfoliation or pigment dispersion component) and/or ocular hypertension who, in the investigator's opinion, were insufficiently controlled on monotherapy or already on multiple IOP-lowering medicinal products, and who had mean baseline diurnal IOP of 26 mmHg, the mean diurnal IOP-lowering effect of SIMBRINZA dosed twice daily was approximately 8 mmHg. Statistically superior reductions in the mean diurnal IOP were observed with SIMBRINZA compared to brinzolamide 10 mg/ml or brimonidine 2 mg/ml dosed twice daily at all visits throughout the study (Figure 1).
Figure 1. Mean
a Diurnal (9 AM, +2 Hrs, +7 Hrs) IOP Change from Baseline (mmHg)—Contribution of Elements Study
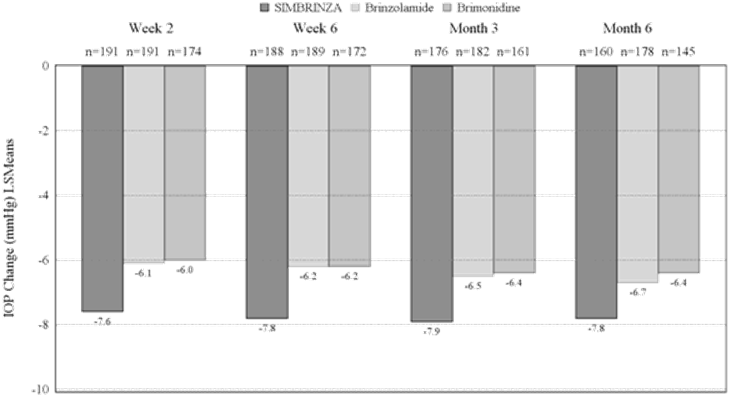 a
aLeast squares means derived from a statistical model that accounts for study site, 9 AM baseline IOP stratum, and correlated IOP measurements within patient.
All treatment differences (SIMBRINZA versus individual components) were statistically significant with p=0.0001 or less.
Mean IOP reductions from baseline at each time point at each visit were greater with SIMBRINZA (6 to 9 mmHg) than monotherapy with either brinzolamide (5 to 7 mmHg) or brimonidine (4 to 7 mmHg). Mean percent IOP reductions from baseline with SIMBRINZA ranged from 23 to 34%. The percentages of patients with an IOP measurement less than 18 mmHg were greater in the SIMBRINZA group than in the Brinzolamide group at 9 of 12 assessments through Month 6 and were greater in the SIMBRINZA group than in the Brimonidine group at all 12 assessments through Month 6. At the + 2 h time point (the time corresponding to the morning efficacy peak) for the primary efficacy visit at Month 3, the percentage of patients with an IOP less than 18 mmHg was 61.7% in the SIMBRINZA group, 40.1% in the Brinzolamide group, and 40.0% in the Brimonidine group.
In a 6-month, controlled, non-inferiority clinical study enrolling 890 patients with open-angle glaucoma (including pseudoexfoliation or pigment dispersion component) and/or ocular hypertension who, in the investigator's opinion, were insufficiently controlled on monotherapy or already on multiple IOP-lowering medicinal products, and who had mean baseline diurnal IOP of 26 to 27 mmHg, . non-inferiority of SIMBRINZA compared to brinzolamide 10 mg/mL + brimonidine 2 mg/mL dosed concomitantly was demonstrated at all visits throughout the study with respect to mean diurnal IOP reduction from baseline (Table 1).'
Table 1. Comparison of Mean Diurnal IOP (mmHg) Change from Baseline– Non-inferiority Study
Visit
| SIMBRINZA
Meana
| Brinzolamide + Brimonidine
Meana
| Difference
Meana (95% CI)
|
Week 2
| -8.4 (n=394)
| -8.4 (n=384)
| -0.0 (-0.4, 0.3)
|
Week 6
| -8.5 (n=384)
| -8.4 (n=377)
| -0.1 (-0.4, 0.2)
|
Month 3
| -8.5 (n=384)
| -8.3 (n=373)
| -0.1 (-0.5, 0.2)
|
Month 6
| -8.1 (n=346)
| -8.2 (n=330)
| 0.1 (-0.3, 0.4)
|
a Least squares means derived from a statistical model that accounts for study site, 9 AM baseline IOP stratum, and correlated IOP measurements within patient
Mean IOP reductions from baseline at each time point at each visit with SIMBRINZA or the individual components administered concomitantly were similar (7 to 10 mmHg). Mean percent IOP reductions from baseline with SIMBRINZA ranged from 25 to 37%.The percentages of patients with an IOP measurement less than 18 mmHg were similar across study visits for the same time point through Month 6 in the SIMBRINZA and Brinzolamide + Brimonidine groups. At the + 2 h time point (the time corresponding to the morning efficacy peak) for the primary efficacy visit at Month 3, the percentage of patients with an IOP less than 18 mmHg was 65.6% in the SIMBRINZA group and 63.7% Brinzolamide + Brimonidine groups.
Paediatric populationThe European Medicines Agency has waived the obligation to submit the results of studies with SIMBRINZA in all subsets of the paediatric population in the treatment of glaucoma and ocular hypertension (see section 4.2 for information on paediatric use).
5.2 Pharmacokinetic propertiesAbsorptionBrinzolamide is absorbed through the cornea following topical ocular administration. The substance is also absorbed into the systemic circulation where it binds strongly to carbonic anhydrase in red blood cells (RBCs). Plasma concentrations are very low. Whole blood elimination half-life is prolonged (>100 days) in humans due to RBC carbonic anhydrase binding.
Brimonidine is rapidly absorbed into the eye following topical administration. In rabbits, maximum ocular concentrations were achieved in less than one hour in most cases. Maximum human plasma concentrations are < 1 ng/mL and achieved within < 1 hour. Plasma levels decline with a half-life of approximately 2-3 hours. No accumulation occurs during chronic administration.
In a topical ocular clinical study comparing the systemic pharmacokinetics of SIMBRINZA administered two or three times daily to brinzolamide and brimonidine administered individually using the same two posologies, the steady-state whole blood brinzolamide and N-desethylbrinzolamide pharmacokinetics were similar between the combination product and brinzolamide administered alone. Likewise, the steady-state plasma pharmacokinetics of brimonidine from the combination was similar to that observed for brimonidine administered alone with the exception of the twice daily SIMBRINZA treatment group, for which the mean AUC
0-12 hours was about 25% lower than that for brimonidine alone administered twice daily.
DistributionStudies in rabbits showed that maximum brinzolamide ocular concentrations following topical administration are in the anterior tissues such as cornea, conjunctiva, aqueous humour and iris-ciliary body. Retention in ocular tissues is prolonged due to binding to carbonic anhydrase. Brinzolamide is moderately bound (about 60%) to human plasma proteins.
Brimonidine exhibits affinity for pigmented ocular tissues, particularly iris-ciliary body, due to its known melanin binding properties. However, clinical and non-clinical safety data show it to be well-tolerated and safe during chronic administration
BiotransformationBrinzolamide is metabolized by hepatic cytochrome P450 isozymes, specifically CYP3A4, CYP2A6, CYP2B6, CYP2C8 and CYP2C9. The primary metabolite is N-desethylbrinzolamide followed by the N-desmethoxypropyl and O-desmethyl metabolites as well as an N-propionic acid analog formed by oxidation of the N-propyl side chain of O-desmethyl brinzolamide. Brinzolamide and N-desethylbrinzolamide do not inhibit cytochrome P450 isozymes at concentrations at least 100-fold above maximum systemic levels.
Brimonidine is extensively metabolized by hepatic aldehyde oxidase with formation of 2-oxobrimonidine, 3-oxobrimonidine and 2,3-dioxobrimonidine being the major metabolites. Oxidative cleavage of the imidazoline ring to 5-bromo-6-guanidinoquinoxaline is also observed.
EliminationBrinzolamide is primarily eliminated in urine unchanged. In humans, urinary brinzolamide and N-desethylbrinzolamide accounted for about 60 and 6% of the dose, respectively. Data in rats showed some biliary excretion (about 30%), primarily as metabolites.
Brimonidine is primarily eliminated in the urine as metabolites. In rats and monkeys, urinary metabolites accounted for 60 to 75% of oral or intravenous doses.
Linearity/non-linearityBrinzolamide pharmacokinetics are inherently non-linear due to saturable binding to carbonic anhydrase in whole blood and various tissues. Steady-state exposure does not increase in a dose-proportional manner.
In contrast, brimonidine exhibits linear pharmacokinetics over the clinically therapeutic dose range.
Pharmacokinetic/pharmacodynamic relationship(s)SIMBRINZA is intended for local action within the eye. Assessment of human ocular exposure at efficacious doses is not feasible. The pharmacokinetic/pharmacodynamic relationship in humans for IOP-lowering has not been established.
Other special populationsStudies to determine the effects of age, race, and renal or hepatic impairment have not been conducted with SIMBRINZA. A study of brinzolamide in Japanese versus non-Japanese subjects showed similar systemic pharmacokinetics between the two groups. In a study of brinzolamide in subjects with renal imapirment, a 1.6- to 2.8-fold increase in the systemic exposure to brinzolamide and N-desethylbrinzolamide between normal and moderately renally-impaired subjects was demonstrated. This increase in steady-state RBC concentrations of substance-related material did not inhibit RBC carbonic anhydrase activity to levels that are associated with systemic side effects. However, the combination product is not recommended for patients with severe renal impairment (creatinine clearance < 30 mL/minute).
The C
max, AUC and elimination half-life of brimonidine are similar in elderly (>65 years of age) subjects compared to young adults. The effects of renal and hepatic impairment on the systemic pharmacokinetics of brimonidine have not been evaluated. Given the low systemic exposure to brimonidine following topical ocular administration, it is expected that changes in plasma exposure would not be clinically relevant.
Paediatric populationThe systemic pharmacokinetics of brinzolamide and brimonidine, alone or in combination, in paediatric patients have not been studied.
5.3 Preclinical safety dataBrinzolamideNon-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity and carcinogenic potential.
Effects in non-clinical reproduction and development toxicity studies were observed only at exposures considered sufficiently in excess of the maximum human exposure indicating little relevance to clinical use. In rabbits, oral, maternally toxic, doses of brinzolamide of up to 6 mg/kg/day (261 times the recommended daily clinical dose of 23 µg/kg/day) revealed no effect on foetal development. In rats doses of 18 mg/kg/day (783 times the recommended daily clinical dose), but not 6 mg/kg/day, resulted in slightly reduced ossification of skull and sternebrae of foetuses. These findings were associated with metabolic acidosis with decreased body weight gain in dams and decreased foetal weights. Dose related decreases in foetal weights were observed in pups of dams given 2 to 18 mg/kg/day. During lactation, the no adverse effect level in the offspring was 5 mg/kg/day.
BrimonidineNon-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, carcinogenic potential, toxicity to reproduction and development.
6. Pharmaceutical particulars6.1 List of excipientsBenzalkonium chloride
Propylene glycol
Carbomer 974P
Boric acid
Mannitol
Sodium chloride
Tyloxapol
Hydrochloric acid and/or sodium hydroxide (to adjust pH)
Purified water
6.2 IncompatibilitiesNot applicable.
6.3 Shelf life2 years.
4 weeks after first opening.
6.4 Special precautions for storageThis medicinal product does not require any special storage conditions.
6.5 Nature and contents of container8 mL round opaque low density polyethylene (LDPE) bottles with a LDPE dropper tip and white polypropylene screw cap (Drop-Tainer) containing 5 mL suspension.
Carton containing 1 or 3 bottles.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handlingNo special requirements for disposal.
7. Marketing authorisation holderAlcon Laboratories (UK) Ltd
Frimley Business Park
Frimley, Camberley
Surrey GU16 7SR
United Kingdom
8. Marketing authorisation number(s)EU/1/14/933/001-002
9. Date of first authorisation/renewal of the authorisationDate of first authorisation: 18
th July 2014
10. Date of revision of the textDetailed information on this medicinal product is available on the website of the European Medicines Agency http://www.ema.europa.eu.
Company contact detailsAlcon Laboratories (U.K) Limitedwww.uk.alcon.com Address
AddressFrimley Business Park, Frimley, Camberley, Surrey, GU16 7SR, UK
Medical Information Direct Line+44 (0) 345 266 9363
Customer Care direct line+44 (0) 8713 760 084